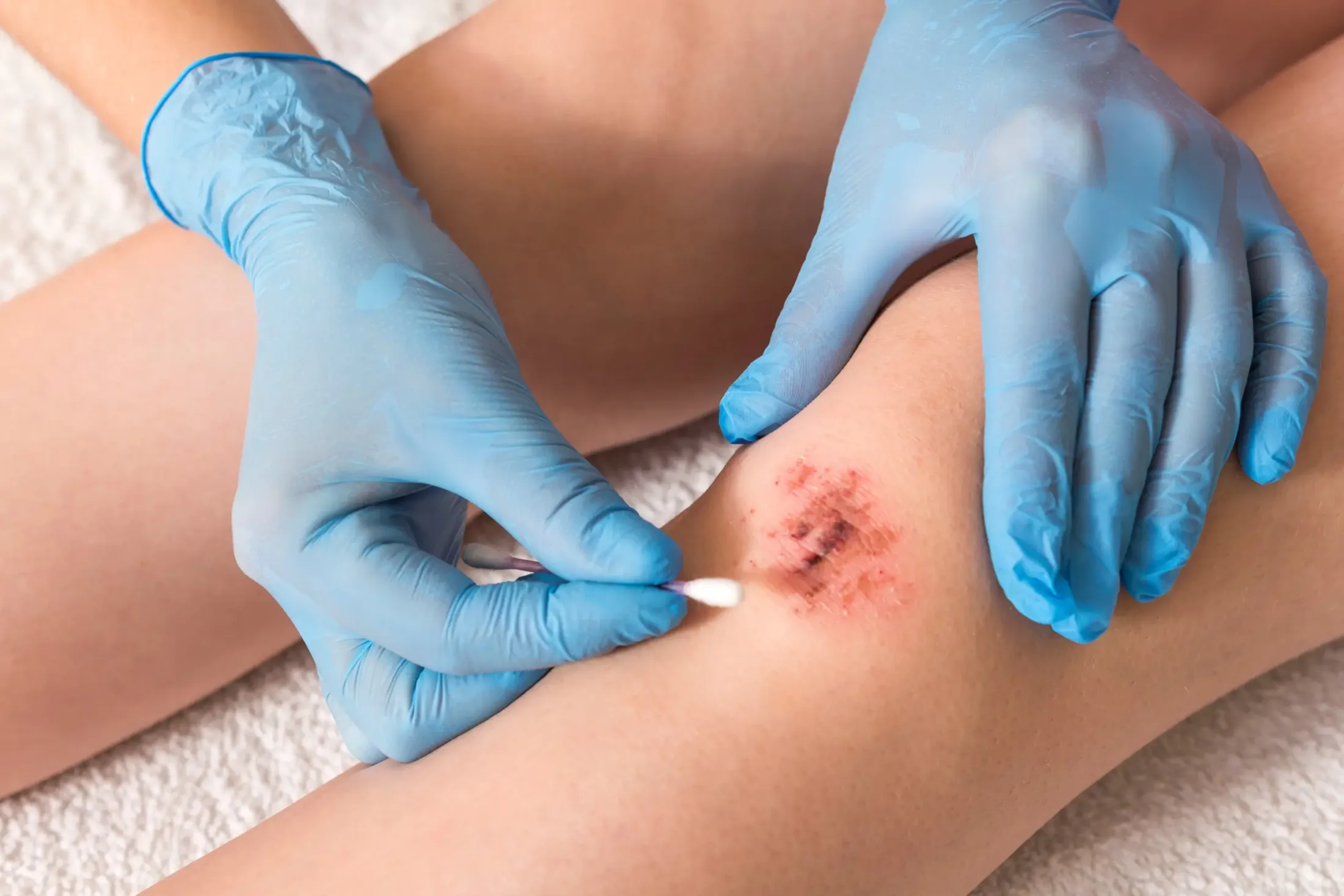
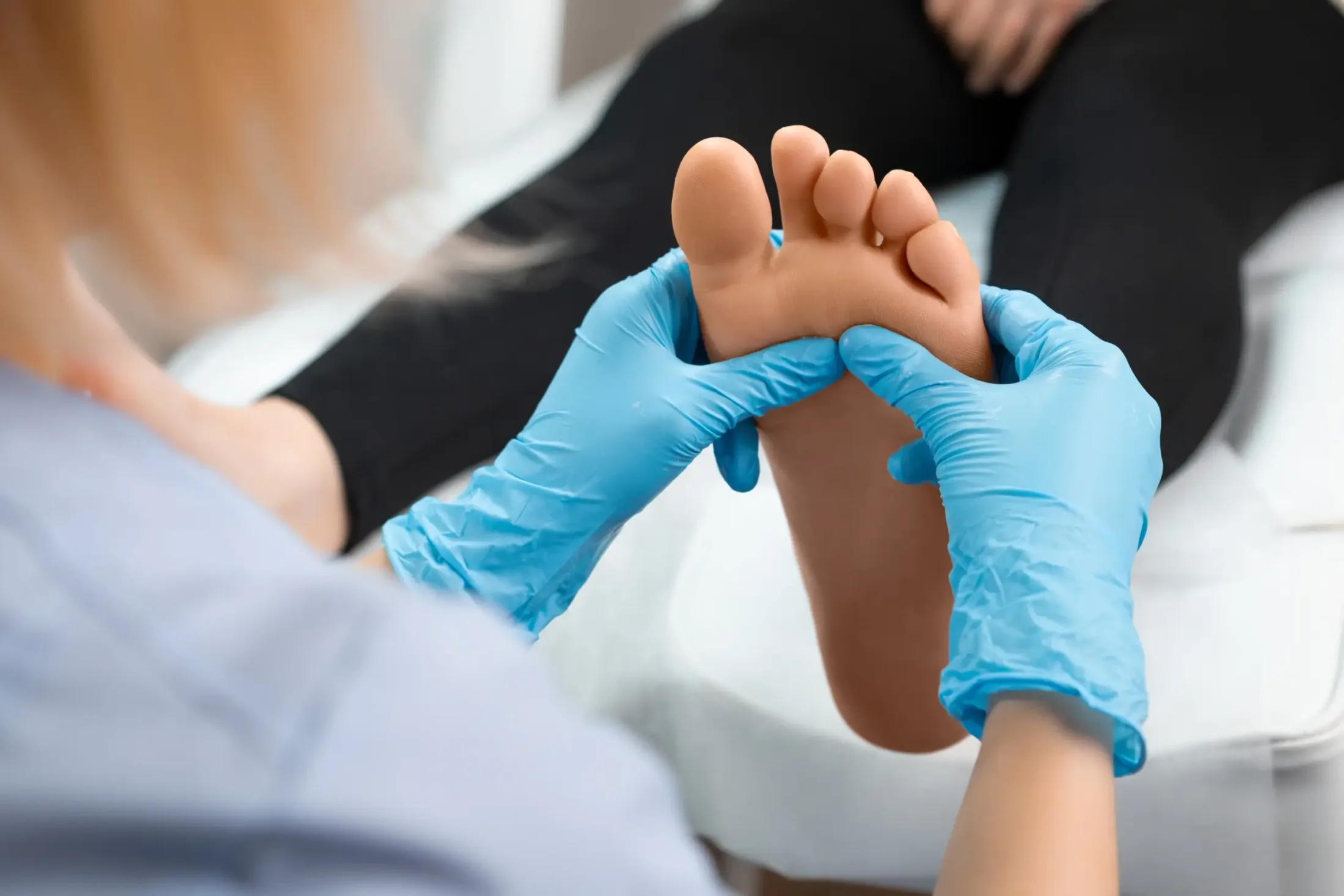
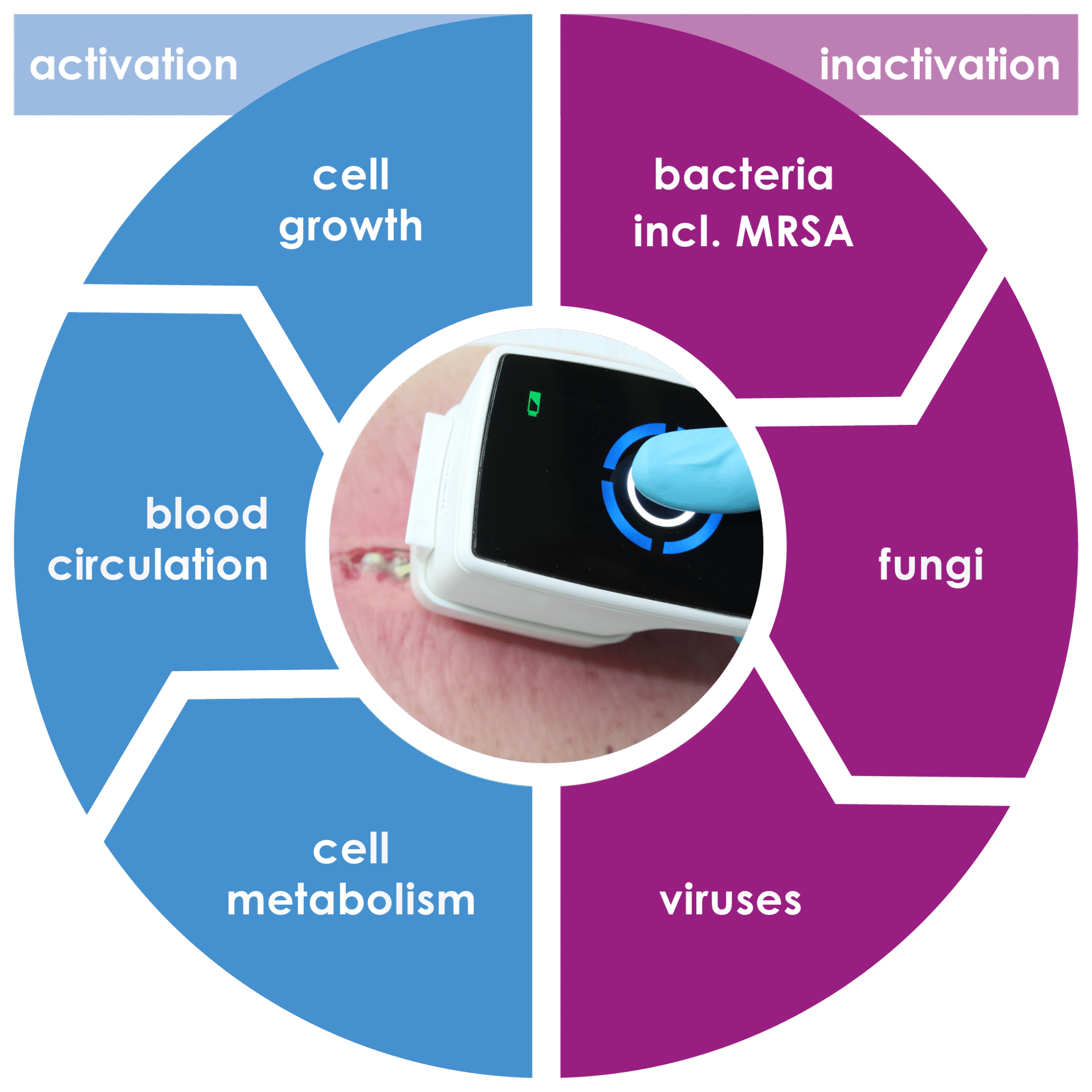
The plasma care® product range inactivates bacteria including multi-resistant pathogens, viruses, fungi and spores. At the same time, it activates the healing process. Over 30,000 successful wound treatments have already been carried out with mobile plasma care®.
The plasma care® product line consists of handy, portable medical devices that generate cold atmospheric plasma from the ambient air.
It uses cold plasma wherever bacteria, viruses and fungi can multiply.
Great technology in a handy device
What is Cold Plasma Treatment?
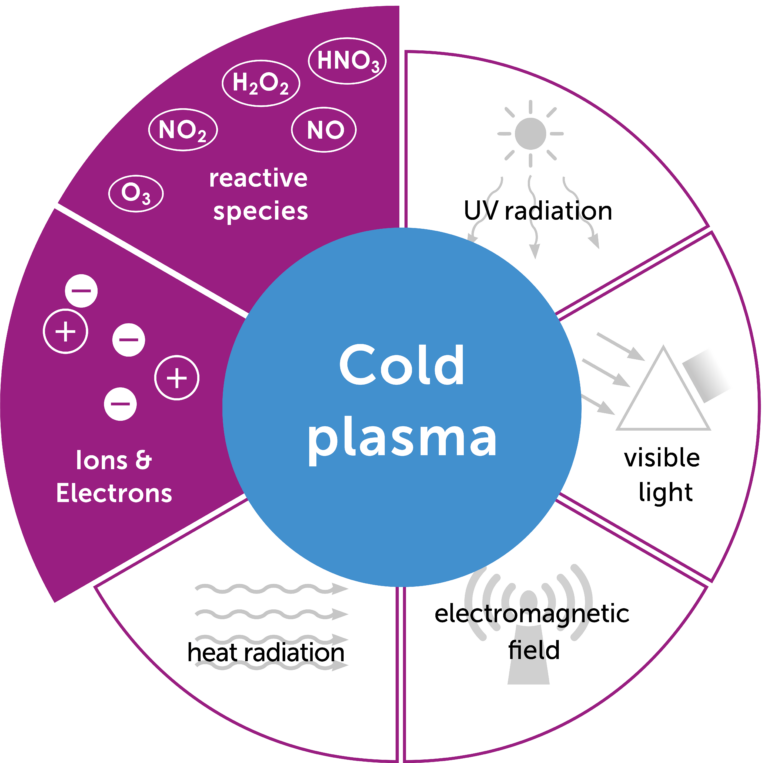
Cold atmospheric plasma is a partially ionized gas at body temperature. When energy is supplied, the so-called plasma cocktail is created. This is poisonous for bacteria, including multi-resistant pathogens, and stimulates cell division in healthy human cells. The plasma activates wound healing and promotes tissue renewal.
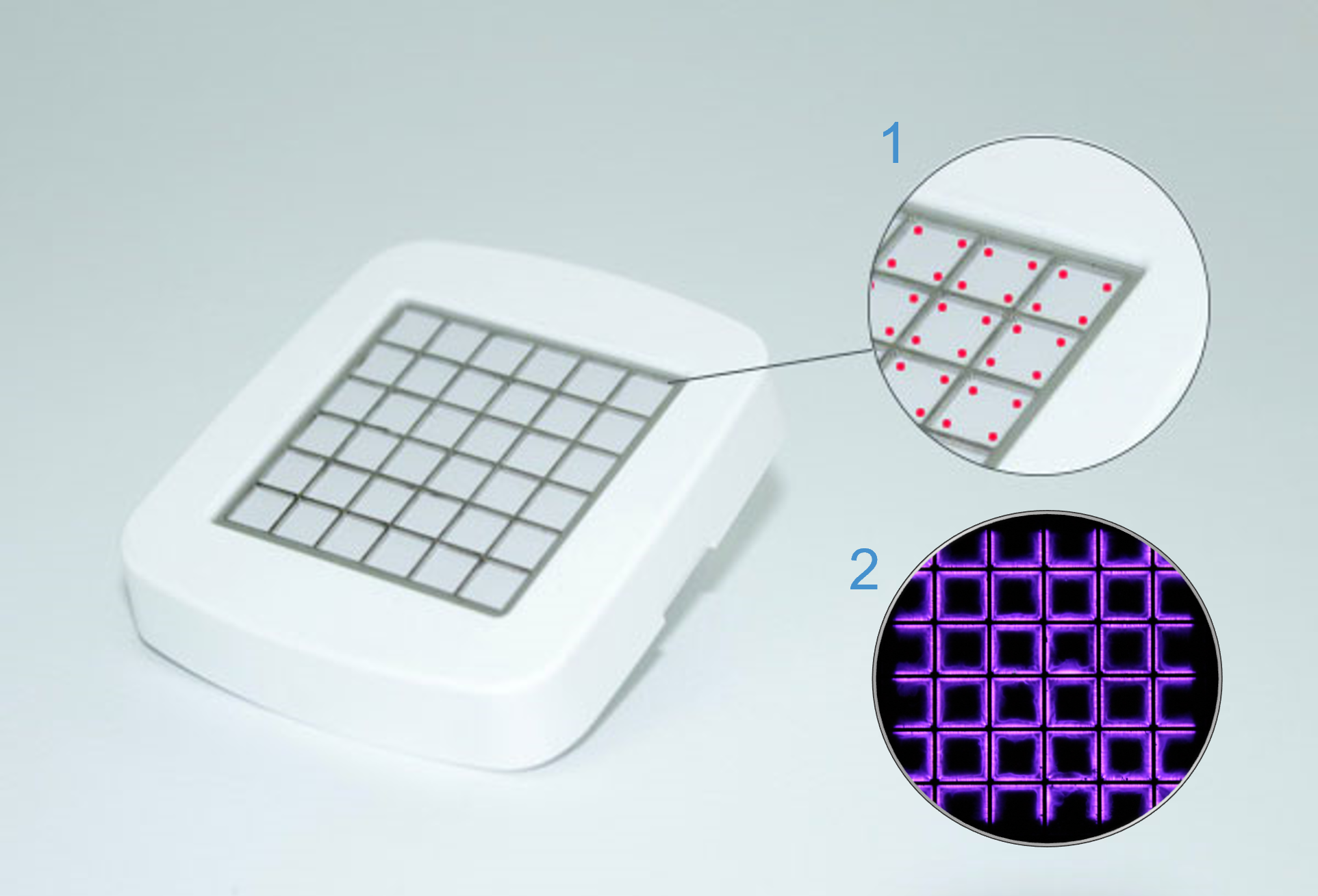
The photo shows an image of a plasma source. 1: Micro discharge in the electrode 2: Plasma source in a darkened environment in which the characteristic purple glow of the plasma is visible.
It brings lightning to body temperature
Plasma is an ionized gas, i.e. it contains charged particles. It is formed from a gas to which energy is added, usually in the form of heat, and is often referred to as the fourth state of matter. Natural examples of plasmas are the sun or lightning on earth.
Cold atmospheric plasmas (CAP) are partially ionized gases, i.e. only one particle of 1∙109 is ionized. The advantage of these cold atmospheric plasmas is that they can exist at body temperature and atmospheric pressure. The plasma care® uses patented thin-film technology, a further development of surface micro-discharge technology (SMD). The plasma source unit consists of a high-voltage electrode, a dielectric and the earthed structured electrode. By applying a high voltage of 3.5 kV, micro-discharges with an extension of a few millimeters occur in the electrode, which is structured in squares. These ionize the gas and thus generate the plasma.
Plasma design
The composition of a cold atmospheric plasma is not a fixed variable, but can be influenced by the choice of plasma source and its operating parameters, among other things. This is referred to as “plasma design”. In the case of SMD cold plasma technology, which is used in plasma care® for wound treatment, the plasma design was selected in such a way that a high bactericidal effect is achieved in vitro and tissue damage is avoided.
Similar to Shimizu et al. 2017 DOI: 10.1615/PlasmaMed.2017019455, tests were carried out with the plasma care® plasma source in oxygen and nitrogen mode to determine the optimum operating parameters for maximum effectiveness and safety. With the help of these and extensive further investigations, the plasma care® was carefully developed for its intended purpose.